Why the US Leaving the World Health Organization is Short-Sighted
On 22 January 2026, the United States announced that it was formally withdrawing from the World Health Organization. Public health experts, analysts, scientists, and those of us who work in the field find this a dangerous decision that will jeopardize national and global security and is scientifically reckless.
This week in the Guardrail, what it looks like when a major player walks away from a seat at the global health table and what that power vacuum actually means for the pharmaceutical industry's bottom line. Especially for businesses trying to stay competitive in a connected world.
By Michelleanne Bradley and Michael Bronfman, Metis Consulting Services
February, 2, 2026
On 22 January 2026, the United States announced that it was formally withdrawing from the World Health Organization. Public health experts, analysts, scientists, and those of us who work in the field find this a dangerous decision that will jeopardize national and global security and is scientifically reckless.
Leaving the World Health Organization is more than a political decision. The consequences are practical, measurable, and deeply connected to health, safety, and a stable economy. This decision weakens disease surveillance, slows drug development, raises health risks, and reduces US influence at a time when worldwide cooperation is imperative.
What the World Health Organization Does
The World Health Organization coordinates global public health efforts. It tracks infectious diseases, sets international health standards, supports vaccination programs, and helps countries respond to emergencies, including pandemics, natural disasters, and outbreaks of emerging diseases.
The WHO's role in medicine, quality, and safety is significant. It runs global systems that monitor drug shortages, counterfeit medication, and adverse drug reactions. These systems support regulators like the US Food and Drug Administration and help pharmaceutical companies operate safely across borders.
The mission of the WHO is to promote health, keep the world safe, and serve the vulnerable.
The US needs the WHO as much as the WHO needs the US.
How WHO Membership Protects the US
Many in the US assume that global health work benefits only other countries. In reality, WHO programs are a first line of defense for the United States.
Disease outbreaks do not respect borders. Viruses travel by plane faster than governments can react. The WHO operates a global disease surveillance network that alerts countries to new threats early, giving US health agencies and pharmaceutical companies time to prepare diagnostics and treatments. Without direct access and influence, the United States risks slower warnings and less reliable information.
During outbreaks such as Ebola, Zika, and COVID-19, WHO data-informed US public health decisions and supported early research efforts.
Currently, US companies are world leaders in medical research and diagnostics, and the WHO is a massive buyer of those goods. In 2023 alone, the WHO purchased over $600 million in US products. When the US is an active member of WHO, contributing to the stability of the global health market, we help prevent mass economic shutdowns. Interruptions to the supply chain, like those that cost the US trillions of dollars during the COVID-19 pandemic, are a concern. Withdrawal from the WHO creates a leadership vacuum that our rivals will fill. With the US involved, we can be sure that global health standards, norms, and research agendas will be consistent with our national interests.
Historically, the US has provided expertise in eliminating diseases worldwide, including smallpox, and has brought polio to the brink of eradication. U.S.-funded programs targeting HIV/AIDS (PEPFAR), tuberculosis, and malaria rely on the WHO’s coordination to be effective. By disengaging from these efforts, we risk collapsing the infrastructure we have built. This can lead to a resurgence of diseases we have spent decades and billions of dollars fighting to suppress.
Impact on Pharmaceutical Research and Drug Development
The pharmaceutical industry depends on worldwide coordination of clinical trials. Regulatory standards rely on shared frameworks. Safety signals are detected through international data sharing.
The WHO supports the development of harmonized guidelines for clinical research, manufacturing quality, and pharmacovigilance. These guidelines reduce duplication, lower costs, and speed patient access to new therapies.
When the US withdrew, companies lost a seat at the table where these standards are shaped. Other countries will still move forward, led by Europe or China, and US firms will then face rules they did not help design. Agencies, including the CDC and NIH, have been instructed to halt official collaboration with the WHO, including co-authoring technical papers and participating in coordinated clinical trials, which previously helped US scientists quickly test treatments in diverse populations. This will create friction in drug development, increase compliance costs, and delay product launches.
Effects on Drug Safety and Quality
The WHO estimates that one in ten medical products in low and middle-income countries is falsified or substandard. These products do not stay overseas. Global supply chains mean unsafe medicines can enter the US market through imports, online pharmacies, or contaminated raw materials.
The WHO helps countries detect and stop these products before they spread. The WHO shares alerts with national regulators, including the FDA. Leaving the WHO weakens the safety net, putting US patients at greater risk of receiving ineffective or dangerous medicines.
Pandemic Preparedness and National Security
The US government has repeatedly recognized pandemics as serious threats to the economy and defense.
The WHO coordinates pandemic preparedness plans, emergency stockpiles, and rapid response teams. It helps countries share virus samples and critical research data for vaccine development. During COVID-19, early sharing of genetic sequences allowed US companies to begin vaccine development within days. That speed saved innumerable lives. Walking away from the WHO does not make the United States more independent. It makes it more isolated at precisely when cooperation matters most.
On 23 January 2026, California became the first US state to join a WHO-coordinated international network independently. California has joined the Global Outbreak Alert and Response Network (GOARN). California accessed international expertise and data for disease monitoring, allowing the state to stay connected to global health security and utilize early warning systems.
Loss of Leadership
For decades, the United States has helped shape global health policy through the WHO. This influence helped to align global health goals with values such as integrity, transparency, and accountability. The US's leaving does not eliminate the WHO; it creates a leadership vacuum. Other nations step in and shape priorities in their stead.
Without US participation, our perspectives on data sharing, regulatory science, and ethical research lose impact. This shift affects everything from outbreak reporting to drug approval standards.
Economic Consequences for the United States
Global health stability supports global economic balance. Outbreaks play havoc with supply chains, reduce workforce productivity, and slow trade. The World Bank estimates that pandemics can cost the global economy trillions of dollars. When the US invests in global disease prevention through organizations like the WHO, it reduces the risk of expensive disruptions that negatively impact businesses and workers.
Future of Global Health
Globally, health challenges are becoming more complex. Climate change is expanding the range of infectious diseases. Antibiotic resistance is rising. New viruses continue to emerge.
No single country can manage these risks alone. The WHO remains the only organization with the reach and mandate to coordinate a global response.
Renewing trust and reforming international institutions are difficult, and abandoning them is not a solution. Active participation allowed the United States to push for transparency and efficiency from within.
Leaving the World Health Organization is a mistake with real consequences. It weakens disease surveillance, slows pharmaceutical innovation, increases safety risks, and reduces US leadership worldwide.
For patients, it means greater exposure to health threats and fewer protections. For the pharmaceutical industry, it means higher uncertainty and reduced influence. For the world, it means a less coordinated response to crises that affect everyone.
Global health cooperation is not a favor to other countries. It is an investment in safety, prosperity, and leadership. Walking away from the WHO does not make the United States stronger. It makes the world, including the US, more vulnerable.
While the current administration maintains that leaving the WHO restores accountability for US taxpayers and allows more autonomous health policy, this is actually a penny-wise, billion-dollar-foolish move, leaving the US more vulnerable to the inevitable return of transnational health threats.
Connect with Metis Consulting Services today to keep your business steady while the global stage shifts. hello@meticconsultingservices.com
Are Your Quality Systems Inspection Ready?
An excellent and sustainable quality management system is the heartbeat of pharmaceutical safety and long-term compliance. This week in the Guardrail, we examine the shift from reactive audit preparation to a proactive culture of regulatory excellence.
An excellent and sustainable quality management system is the heartbeat of pharmaceutical safety and long-term compliance. This week in the Guardrail, we examine the shift from reactive audit preparation to a proactive culture of regulatory excellence.
By Michael Bronfman, for Metis Consulting Services
January 26, 2026
Quality systems are essential for every pharmaceutical company. Quality Assurance and Quality Control ensure that drugs are developed, manufactured, and distributed safely and in compliance with regulations. Inspection readiness means more than just passing an FDA or EMA inspection; it involves building a culture of quality that supports compliance, efficiency, and public trust.
Regulators expect companies to have strong quality systems, and inspections test how well these systems work in practice. Companies that start preparing only after receiving an inspection notice run into problems that could have been avoided with earlier preparation.
Understanding Quality Systems
A quality system is a group of policies, procedures, and practices that help products consistently meet requirements. It includes document control, change management, handling deviations, corrective and preventive actions, audits, and training.
The FDA provides guidance on quality systems through regulations such as 21 CFR Parts 210 and 211 for pharmaceuticals and Part 820 for medical devices.
Information is available at https://www.fda.gov/industry.
The EMA also guides good manufacturing practice and quality system expectations at Good Manufacturing Practice | European Medicines Agency (EMA)
Understanding the regulatory framework is the first step toward being ready for inspections.
Inspection Readiness Is Continuous
Inspection readiness is not a one-time task; it is part of everyday work. Internal and external (consultants, contractors, and vendors) staff need training on procedures and are expected to understand how their work supports overall quality.
Key components of inspection readiness include:
Up-to-date documentation: Standard operating procedures, batch records, validation protocols, and training records should always be current and complete.
Traceability: Actions and decisions need documentation so any process can be followed from start to finish.
Accountability: Roles and responsibilities should be clear, and staff should be able to show they understand their tasks.
Continuous monitoring: Metrics and trends are expected to be reviewed regularly to identify potential issues before they worsen.
Conducting Self Assessments
Self-assessments, or internal audits, are one way to prepare for inspections. They help to identify gaps, verify compliance, and offer staff a chance to practice answering regulator questions.
To make self-assessments and process reviews effective, review each quality system process to ensure it complies with procedures and regulations. Audits of records include randomly selected records to check for accuracy, completeness, and timeliness, mock inspections, where a regulatory inspection is simulated, help train staff, and identify weak areas. Any issues identified during self-assessments are to be remedied through corrective and preventive actions. Taking this proactive approach reduces risk during an actual inspection.
Document Control and Data Integrity
Document control is central to every quality system. SOPs, training records, batch records, validation documents, and audit reports are always to be current, well-organized, and easy to access.
Data integrity is crucial. Regulators expect records to be accurate, complete, and protected from unauthorized changes.
FDA guidance on data integrity and compliance is available at https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations
Maintaining strong document control and data integrity builds trust in the quality system and helps with inspection readiness.
Training and Competency
Staff training goes beyond completing courses. Employees should understand their roles, know the procedures, and demonstrate competency.
Training programs should include:
Initial onboarding on quality system expectations
Role-specific technical training
Refresher training for updates to procedures or regulations
Records demonstrating completion and comprehension
Skilled and confident staff play a significant role in successful inspections.
Corrective and Preventive Actions
No system is perfect, so deviations and nonconformities will happen. How a company responds shows the strength of its quality system. Active CAPA programs include:
Root cause analysis: Find the real reason for the issue, not just the symptom. Effective action: Address the specific issue promptly and completely
Preventive action: Make changes to stop the problem from happening again. Confirm that the CAPA has resolved the issue and improved the process.
Inspectors look closely at CAPA records. Well-documented CAPAs demonstrate that the company is proactive and compliant with regulations.
Audit Programs
Internal and external audits are essential for ongoing improvement. Regular internal audits help find gaps and improve processes. Vendor audits ensure suppliers meet quality standards and regulatory requirements.
Audits should include documented findings, follow-up actions, and checks to confirm that changes were effective. A robust audit program shows regulators that quality is actively managed.
Managing Regulatory Inspections
When an FDA or EMA inspection takes place, being prepared makes a big difference. Key steps include:
Leadership involvement: Make sure managers are visible and know what is going on.
Document accessibility: Keep records organized so they can be found quickly.
Staff readiness: Train staff to answer questions with facts and confidence.
Issue resolution: Set up a process to document follow-up and respond to any observations. Inspectors look at both compliance and the company’s culture of quality and ongoing improvement.
Leveraging Technology
Quality Systems are encouraged to use technology to work more efficiently and track progress. Electronic quality management systems (eQMS) help with document control, CAPA tracking, training management, and audit programs. Using technology properly improves traceability, reduces errors, and makes inspection preparation easier. It also helps monitor trends and risks.
Continuous Improvement
A quality system that is always ready for inspection is continuously evolving and improving. Continuous improvement helps processes develop, closes gaps, and uses lessons from audits, assessments, and daily work.
Reviewing metrics and trends, and comparing them to industry standards, helps maintain high performance. Companies that focus on continuous improvement are better prepared for inspections and achieve better quality results.
Looking Ahead
Inspection readiness is not just a checklist. It is an ongoing commitment to quality, compliance, and patient safety. Integrating training, documentation, monitoring, and continuous improvement into daily work, companies can reduce risk and demonstrate regulatory excellence, transparency, and accountability.
Organizations that embrace these expectations and maintain strong quality systems will be able to respond confidently to inspections, protect patients, and sustain long-term success.
Ready to transform your compliance strategy? Contact Metis Consulting Servicestoday to schedule a consultation with our team of experts.
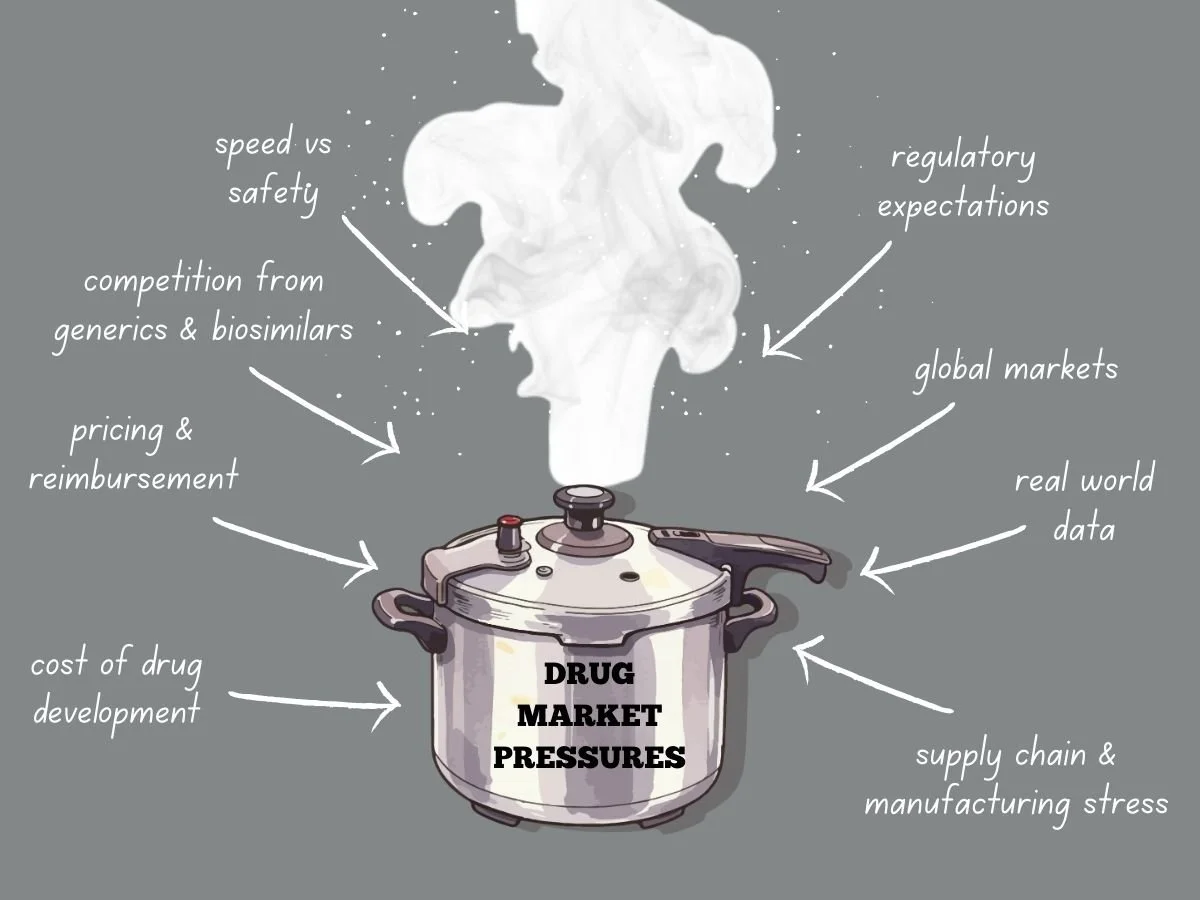
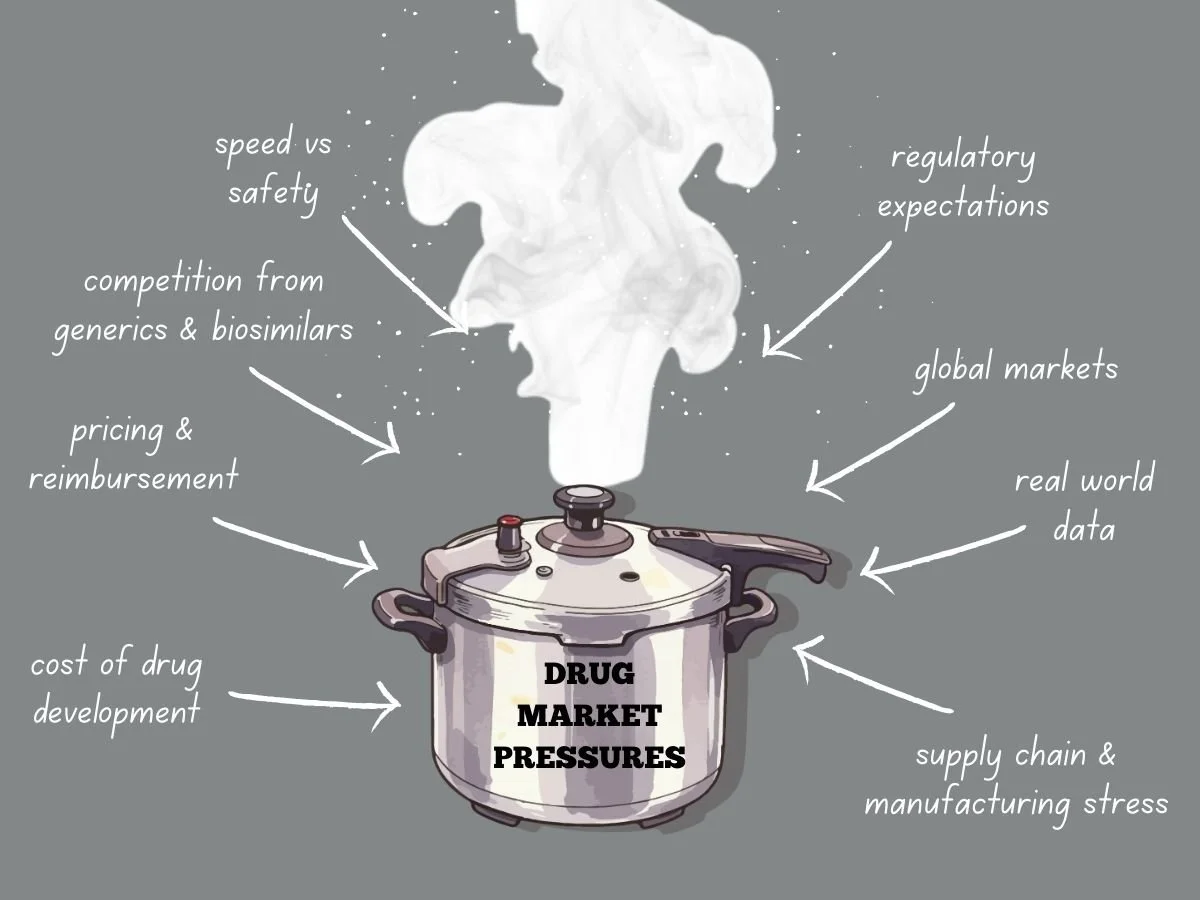
Navigating Intense Drug Market Pressures
Today’s global drug market faces more pressure than ever before. Pharmaceutical companies deal with higher development costs, tougher regulations, stricter pricing, and more competition from generics and biosimilars. Meanwhile, patients, payers, and governments want quicker access to safe and effective treatments. These factors are changing how companies plan, develop, launch, and manage products over time.
To succeed, companies need to know what causes market pressure and how it shapes their decisions. They also have to adjust their strategies while keeping quality, safety, and compliance intact.
This week in the Guardrail, we explore the intensifying economic and regulatory forces reshaping the global pharmaceutical landscape. We analyze the ways industry leaders can maintain compliance and quality while navigating the high-stakes pressures of modern drug development.
By Michael Bronfman for Metis Consulting Services
January 19, 2026
Today’s global drug market faces more pressure than ever before. Pharmaceutical companies deal with higher development costs, tougher regulations, stricter pricing, and more competition from generics and biosimilars. Meanwhile, patients, payers, and governments want quicker access to safe and effective treatments. These factors are changing how companies plan, develop, launch, and manage products over time.
To succeed, companies need to know what causes market pressure and how it shapes their decisions. They also have to adjust their strategies while keeping quality, safety, and compliance intact.
The Cost Reality of Drug Development
Developing new drugs is both costly and risky. It can take billions of dollars to bring a new medicine to market, especially when you include failed attempts. Clinical trials last for years, regulatory submissions need a lot of data, and manufacturing must meet rigorous quality standards.
As development costs go up, so does market pressure. Investors want to see returns, so leaders have to focus on programs most likely to succeed. This means making tough choices about which therapies to continue and which to pause or stop.
Pressure grows when competitors work on similar products. If a company is second to market, it can lose pricing power and market share. This pushes companies to find ways to develop products faster while still following the rules.
Pricing and Reimbursement Challenges
Pricing pressure is a major challenge in today’s drug market. Governments and private payers are resisting high launch prices, and value-based pricing is becoming more common. Companies now have to clearly show clinical benefits, real-world results, and economic value.
In the United States, pricing scrutiny continues to grow through policy changes and public debate. Programs like Medicare negotiation place additional pressure on manufacturers to justify pricing decisions. The NIH National Library of Medicine is a good resource for more information at https://pmc.ncbi.nlm.nih.gov/articles/PMC11129567/
In Europe, pricing and reimbursement decisions are often made at the country level. Health technology assessments play a significant role in determining whether a product will be reimbursed and at what price. The European Medicines Agency provides regulatory approval, but market access depends on additional reviews. https://globalpricing.com/pricing-and-reimbursement-trends-in-europe-current-landscape-and-implications/
Because of these pressures, companies can not wait until after approval to plan for market access. They need to start early, thinking about evidence, comparators, and patient groups.
Competition from Generics and Biosimilars
Patent expiration is still a major source of market pressure. Once exclusivity ends, generics and biosimilars can quickly cut into revenue. Sometimes, prices fall by over 80 percent in the first year.
Companies have to plan their product life cycles early. They might consider new formulations, more uses, or combination products. Each choice needs careful regulatory planning and strong supporting data.
The US Food and Drug Administration provides guidance on generics and biosimilars, including approval pathways and exclusivity considerations at https://www.fda.gov/drugs/development-approval-process-drugs/how-drugs-are-developed-and-approved.
Companies that wait too long to plan for the loss of exclusivity often have trouble protecting their product’s value when competitors arrive.
Speed Versus Safety
Market pressure often makes teams move faster. Quicker development can help patients get treatments sooner and improve a company’s position. But moving too fast without proper controls can lead to big risks.
Rushing trials can lead to poor study design or trouble enrolling patients. If data is incomplete, it can cause regulatory delays or extra work after approval. Cutting corners in manufacturing can cause quality problems and require more inspections.
To balance speed and safety, companies need strong oversight. Clear decision rules, teamwork across departments, and early talks with regulators are key. Programs that focus on quality from the beginning handle market pressure better.
Regulatory Expectations Do Not Ease Under Pressure
One common misconception is that regulators may be more flexible when market needs are urgent. While there are faster review programs, the standards for safety, effectiveness, and quality do not change for the Fast Track and Breakthrough Therapy designation. These programs aim to speed access while maintaining standards.
The FDA provides guidance:
But it can be tricky to navigate these expectations.
Using these pathways successfully requires careful planning and ongoing communication with regulators. Market pressure is not a good reason for weak data or incomplete submissions. This adds another layer of pressure. Regulatory requirements vary by region. Clinical trial designs must support multiple agencies. Manufacturing and labeling must meet diverse standards.
If regions are not aligned, it can cause delays and extra costs. For example, a trial designed just for the US might not work in Europe or Asia. Aligning global strategy early helps avoid these problems.
The International Council for Harmonisation plays a key role in aligning technical requirements across regions. Information on these guidelines is available at https://www.ich.org/page/search-index-ich-guidelines.
Understanding and applyLearning and using these guidelines early helps companies handle global market pressure better.
Global Markets Add Complexity
Many products are developed for global markets. This adds another layer of pressure. Regulatory requirements vary by region. Clinical trial designs must support multiple agencies. Manufacturing and labeling must meet diverse standards.
Misalignment between regions can lead to delays and added costs. For example, a trial designed only to meet US requirements may fall short in Europe or Asia. Early global strategy alignment helps reduce this risk.
The International Council for Harmonisation plays a key role in aligning technical requirements across regions. Information on guidelines is available at https://www.ich.org/page/search-index-ich-guidelines
Understanding and applying these guidelines early helps companies manage global market pressure more effectively.
Manufacturing Stress
Market pressure continues after approval. Manufacturing and supply chains have their own problems. It’s hard to predict demand, especially for new products. Shortages, global issues, and quality problems can all disrupt supply. Manufacturers to maintain control and continuity of supply. Inspections focus on data integrity, process validation, and change management. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations
When under a lot of pressure, companies might try to stretch their capacity or put off investments. These choices often increase risk and can lead to regulatory problems.
The Role of Real World Evidence
As pricing and access decisions rely more on data, real-world evidence is becoming more important. Payers and regulators want to know how products work outside of controlled trials.
To collect good real-world data, companies need planning, the right systems, and oversight. They also have to meet regulatory standards for data reliability and privacy.
https://www.fda.gov/science-research
Companies that build these capabilities early are better able to handle market pressure and keep their products valuable over time.
Organizational Alignment Under Pressure
Market pressure often reveals weak spots in how organizations are set up. Gaps between clinical, regulatory, quality, and commercial teams can slow decisions. Conflicting goals can also cause teams to lose focus.
Successful organizations set shared goals and maintain open communication. They invest in training and clear processes. Leaders make it clear that compliance and quality always come first, even when deadlines are tight.
This kind of culture is essential when companies face inspections, audits, or public attention.
Looking Ahead
Drug market pressures are not going away. If anything, they will continue to intensify. Companies that treat pressure as a reason to cut corners will face setbacks. Those who use pressure as a driver for more thoughtful planning, stronger execution, and earlier collaboration will be better positioned to succeed.
Navigating this environment requires discipline, foresight, and respect for regulatory expectations. It also requires a clear focus on patients, who remain the ultimate reason these products exist.
The path from laboratory to patient has never been more complex, but you don't have to navigate these regulatory and economic hurdles alone. Contact Metis Consulting Services today. Learn how our strategic oversight and industry expertise can help your organization transform market pressure into a sustainable competitive advantage.
The Rise of Computer-Designed Antibodies and What It Means for Therapeutics
Recently, researchers have developed advanced computational tools to design antibodies in ways previously considered unattainable. This development could accelerate the discovery of new treatments and expand the range of treatable diseases.
As the field of drug discovery undergoes a monumental shift toward computational efficiency, staying ahead of regulatory and quality benchmarks is essential for success. This week in the Guardrail, we examine how the rise of computer-designed antibodies is redefining what is possible for modern therapeutics
By Michael Bronfman for Metis Consulting Services
January 12, 2026
Life sciences are undergoing a significant shift in drug discovery. Historical methods depend on animal testing, extensive molecular libraries, and lengthy trial-and-error cycles. Recently, researchers have developed advanced computational tools to design antibodies in ways previously considered unattainable. This development could accelerate the discovery of new treatments and expand the range of treatable diseases.
An antibody is a type of protein that your immune system makes to recognize and bind to substances such as viruses or bacteria. Because antibodies can bind very specifically to targets, they make excellent therapeutic drugs. There are already many antibody medicines on the market for cancer, autoimmune diseases, and infectious diseases. But creating new antibody drugs with traditional methods is slow, costly, and often unpredictable. Researchers now use advanced computer models to guide antibody design. These models “learn” from large-scale biological datasets to propose new proteins that bind targets with high precision and affinity.
Learn more at the PubMed review on antibody design advances.
In this article, we will explore why computer-designed antibodies are now possible, how they may improve treatments, and what challenges remain.
What Are Antibodies and Why Are They Important
Antibodies are proteins produced by the immune system to recognize and attach to antigens. They look like a “Y” shape with two arms that grab the target. The part of the target that binds the ligand is called the binding region. This region can be fine-tuned to stick firmly to one specific target protein. Many modern drugs are antibodies because they can block harmful proteins without interfering with normal body functions.
More than one hundred therapeutic antibodies have been approved for use in humans. Some of these work by blocking signals that promote cancer-cell growth. Others mark infected cells so the immune system can destroy them. Because these drugs are specific, they often cause fewer side effects than traditional small-molecule drugs. But making new antibody drugs requires many months of lab work. Traditional methods involve immunizing animals or screening large collections of molecules to identify rare, suitable candidates. These processes are expensive and sometimes fail to find suitable matches for challenging targets.
How Computers Can Help Design Antibodies
Computational design changes this process by using large datasets and predictive models to propose antibody candidates before they are made in the lab. These tools examine protein shapes and their interactions. They can then suggest novel antibody sequences that should fold into shapes that bind strongly to a chosen target.
One significant advance came when research groups taught computers to build antibodies from scratch. At the University of Washington, scientists created complete antibodies entirely on computers. They controlled where the antibody would bind and then tested the designs in the lab. Many of these computer designs folded and bound targets as expected. The result suggests computers can help design new antibody drugs much faster than traditional methods.
In addition to full antibody design, other computational tools can optimize specific antibody regions. For example, they can predict how changes in amino acid sequence might increase binding strength or reduce unwanted interactions. The combination of prediction and testing accelerates the full path from idea to experimental candidate.
Examples of Progress in Therapeutic Antibody Design
A recent milestone in this field is Imneskibart (AU-007). This is the first fully computer-designed antibody to enter clinical trials. It was created to bind a specific part of the immune system and modulate immune responses in cancer without causing the common toxic side effects seen with older therapies. The fact that this medicine has reached clinical testing is significant proof of concept for computational design methods.
Another example is in the Reuter’s report on industry partnerships between a U.S. biotech company and a global pharmaceutical firm. They expanded their research collaboration to focus on protein and antibody design using advanced computational platforms. These platforms can propose designs and move them to initial laboratory testing in only a few weeks, compared to months or years with older methods.
Alongside specific antibody drugs, research groups worldwide are using technology to tackle challenging disease targets. That includes chronic infections, rapidly mutating cancer antigens, and proteins previously considered undruggable. These new tools give scientists more control over the design process and reduce reliance on random screening as shown in the Pharmaceutical Journal on the future of antibody drugs.
Benefits of Computer-Designed Antibodies
There are several essential benefits to designing antibodies with computational methods:
1. Speed: Historical discovery can take years. Computational design can quickly narrow down promising candidates and may cut months from early phases of drug discovery.
2. Precision: Computers can predict the exact spot, or epitope, on a target protein where an antibody will bind. This precision helps create drugs that block specific functions without interfering with other parts of the body.
3. Better screening: Instead of testing millions of random molecules, researchers can use computational filters to test just a few dozen promising candidates in the lab. This reduces cost and waste.
4. Hard targets: Some disease targets are very difficult to bind with traditional methods. Computational design can explore new molecular shapes that might succeed where older methods fail.
5. Reduced side effects: By designing antibodies that bind only to intended targets, there is a potential for fewer off-target interactions that cause adverse effects.
In many ways, these new computer-guided tools behave like powerful microscopes. They allow scientists to see and test possibilities that were once invisible or unreachable with older methods.
Learn more at the University of Washington’s report on computer-designed antibodies in nature.
Challenges and Limitations
Even though these new design methods are powerful, they are not yet perfect. A central challenge is validation. Computers can propose many candidate molecules, but only some of these actually fold and bind as predicted in real lab conditions. Researchers still need to test candidates experimentally before they become drug candidates.
Another challenge is that the design models depend on large datasets of known protein structures. If a target is very different from anything in the datasets, the design models may not make accurate predictions. Scientists are working to expand these training sets and improve model performance.
There are also development hurdles. Even after a good candidate is found, it must be manufactured reliably and safely. The pathway from an early design to an approved drug includes multiple steps, including tissue testing, toxicology studies, and clinical trials, which remain costly and time-consuming.
Finally, there is the question of accessibility. Currently, many of the most advanced design tools are available only to large companies or research institutions with significant computing resources. Making these tools more widely available could help smaller organizations contribute to discovery and innovation.
What This Means for Future Medicines
The rise of computer-designed antibodies may change what is possible in medicine. Because these tools speed up early discovery, they could bring new treatments to patients faster than ever before. This could be valuable for diseases that have no good treatments today.
For example, researchers are using computational design to pursue cancer targets that mutate rapidly and immune molecules with complex structures. These targets were once considered too difficult for standard methods. If computers can identify stable designs for these targets, new therapies could reach patients in need.
In addition, the improved precision may lead to safer medicines. With a better understanding of how an antibody binds its target, scientists can avoid unintended effects that cause harm. As computational tools improve and large datasets grow, the accuracy of these predictions will also increase.
Because of the faster pace of design, new antibody treatments could be developed for emerging infectious diseases. During a pandemic or outbreak, the ability to rapidly design antibodies that neutralize a threat could save many lives.
Overall, we are nearing a time when computers are normal parts of the drug discovery toolkit. They do not replace human scientists but give them powerful new tools to explore possibilities that would be very hard to test with old methods.
The growth of computer-designed antibodies shows how technology can reshape life sciences. These tools bring speed, precision, and new possibilities to therapeutic discovery. While challenges remain, the progress so far suggests a future where new treatments can be developed more rapidly and more safely. For patients with unmet medical needs, this change could be life-changing.
The promise of these methods comes from their ability to transform what used to be guesswork into guided design. As computational capabilities continue to improve and merge with experimental science, the pace of discovery will only increase. The future of therapeutics will include more medicines that were first conceived on a computer screen and then tested and refined in the lab.
As your organization adopts cutting-edge technologies like computational antibody design, Accelerate Innovation with Metis Consulting Services. Navigating the complexities of quality, regulatory strategy, and data management is more challenging than ever. We provide the expert guidance you need to transform these technological breakthroughs into safe, market-ready therapies. Contact Metis Consulting Services today to schedule a consultation and ensure your pipeline is built on a foundation of wisdom and precision. hello@metsconsultingservices
Does Coffee Have an Impact on Cholesterol
This article explores how coffee affects cholesterol levels, how different brewing methods change that effect, and what current research suggests is the healthiest way to brew coffee.
For Metis consulting services
By Michael Bronfman
We are looking at your favorite morning beverage again this week at The Guardrail, as we examine the link between coffee preparation methods and cholesterol levels, for heart-conscious consumers.
How Brewing Methods Shape Heart Health
Coffee is one of the most widely consumed beverages in the world. In the United States alone, millions of adults drink coffee every day. Coffee is often praised for its antioxidants and potential benefits for metabolism, brain health, and Type 2 diabetes risk. However, coffee also contains natural compounds that can influence cholesterol levels. Coffee brewing method plays a key role in whether those compounds reach the final cup.
This article explores how coffee affects cholesterol levels, how different brewing methods change that effect, and what current research suggests is the healthiest way to brew coffee.
Understanding Cholesterol and Heart Risk
Cholesterol is a waxy substance found in the blood. The body needs cholesterol to build cells and produce hormones, but too much can increase the risk of heart disease.
Low-density lipoprotein cholesterol is often called "bad cholesterol." High levels of LDL cholesterol can lead to plaque buildup in the arteries. High-density lipoprotein cholesterol, also known as "good cholesterol," helps remove LDL cholesterol from the bloodstream.
Diet plays a significant role in cholesterol levels. Saturated fat, trans fat, and certain food compounds can raise LDL cholesterol. Coffee contains specific compounds that fall into this category depending on its preparation.
Coffee Oils and Their Effect on Cholesterol
Coffee beans naturally contain oils. Two of the most studied compounds in these oils are cafestol and kahweol. These compounds belong to a group called diterpenes.
Research has shown that cafestol and kahweol can raise LDL cholesterol by disrupting the liver's regulation of cholesterol production and clearance. Cafestol in particular is considered one of the most potent cholesterol-raising compounds found in the human diet.
The key factor is whether these coffee oils make it into the cup. Brewing methods that allow oils to pass through result in higher diterpene intake. Brewing methods that remove oils through filtration reduce this effect.
Filtered Coffee and Cholesterol
Filtered coffee uses a paper filter to trap coffee grounds and oils. Common examples include drip coffee makers and pour-over methods that use paper filters.
Paper filters are effective at removing most cafestol and kahweol before the coffee reaches the cup. As a result, filtered coffee has little to no effect on LDL cholesterol for most people.
An extensive observational study published in the European Journal of Preventive Cardiology examined coffee consumption among more than 500,000 adults aged 18 or older. The study found that people who drank filtered coffee had lower rates of heart disease and lower overall mortality compared with those who drank unfiltered coffee.
The authors concluded that filtered coffee was the safest option for cardiovascular health.
https://academic.oup.com/eurjpc/article/27/18/1986/5909512
French Press Coffee and Cholesterol
French press coffee does not use a paper filter. Instead, coffee grounds steep directly in hot water, and a metal mesh plunger separates the liquid from the grounds. This method allows coffee oils to remain in the final beverage.
Studies have consistently shown that French press coffee contains higher levels of cafestol and kahweol. Regular consumption of French press coffee has been linked to increased LDL cholesterol levels, especially when consumed in large amounts.
An early controlled trial published in the New England Journal of Medicine found that drinking unfiltered coffee significantly raised cholesterol levels compared with filtered coffee.
For individuals with elevated cholesterol, heart disease, or a family history of cardiovascular risk, French press coffee may not be the best daily choice.
Espresso and Cholesterol
Espresso is often misunderstood when it comes to cholesterol. Espresso is brewed quickly under pressure and does not use a paper filter. This means coffee oils are present in the final shot.
However, serving size matters. A typical espresso shot is much smaller than a standard cup of drip coffee. As a result, total diterpene intake may be lower even though concentration is higher.
Research suggests that moderate espresso consumption may raise cholesterol slightly but less than larger volumes of unfiltered coffee, such as French press. Drinking several espresso-based drinks per day may still contribute to increased LDL cholesterol over time.
A review published in Current Atherosclerosis Reports noted that espresso contains diterpenes, but the overall impact depends on dose and frequency.
Turkish and Greek Coffee
Turkish and Greek coffee are brewed by boiling finely ground coffee directly in water. The grounds are not filtered out before drinking. This method results in very high diterpene content.
Studies have shown that boiled coffee can raise LDL cholesterol more than any other common brewing method. Regular intake has been associated with increased cholesterol and cardiovascular risk, especially in populations with high consumption.
The same Norwegian study that examined filtered coffee found that people who drank large amounts of unfiltered coffee had higher mortality rates compared with filtered coffee drinkers.
Cold Brew Coffee
Cold brew coffee is made by steeping coffee grounds in cold water for many hours. The grounds are usually removed with a filter or mesh.
Cold-brew prepared with paper filtration likely removes most diterpenes, as does hot filtered coffee. Cold brew made with metal filters may retain more oils.
There is limited research specifically on cold brew and cholesterol. However, experts generally agree that filtration matters more than temperature. Using a paper filter is the safest option for cholesterol control.
Does Coffee Raise or Lower Heart Disease Risk
Coffee contains many biologically active compounds beyond diterpenes. These include antioxidants, polyphenols, and caffeine. Some of these compounds may improve insulin sensitivity, reduce inflammation, and support blood vessel function.
Extensive population studies suggest that moderate coffee consumption is associated with a lower risk of heart disease, stroke, and overall mortality when consumed regularly and without excessive sugar or cream.
The American Heart Association states that moderate coffee intake does not appear to increase cardiovascular risk for most healthy adults. In the article, Is Coffee Good for You or Not?
The key distinction is brewing method. Filtered coffee appears to offer benefits without raising cholesterol, while unfiltered coffee may increase LDL cholesterol and offset potential benefits.
How Much Coffee Is Safe
Most dietary guidelines suggest that up to four hundred milligrams of caffeine per day is safe for healthy adults. This amount is roughly equal to three to five cups of brewed coffee.
People with high blood pressure, heart rhythm disorders, anxiety, or sleep issues may need to limit caffeine regardless of brewing method.
Consistency also matters. Drinking coffee regularly appears to be better tolerated than drinking it sporadically. Sudden intake can raise blood pressure in sensitive individuals.
The Healthiest Way to Brew Coffee
Based on current evidence, the healthiest way to brew coffee for cholesterol and heart health is to use a paper filter.
This method removes most cholesterol-raising compounds while preserving beneficial antioxidants. Drinking filtered coffee without added sugar, flavored syrups, or high-fat creamers further supports cardiovascular health.
For those who enjoy espresso or French press coffee, moderation is key. Limiting intake and balancing with filtered coffee may reduce cholesterol impact.
Individuals with high cholesterol or established heart disease should consider switching to filtered coffee as a simple lifestyle change that may improve lipid levels.
Practical Takeaways for Patients and Clinicians
Coffee can be part of a heart-healthy diet when prepared thoughtfully. Brewing method matters more than many people realize.
Filtered coffee is associated with lower cholesterol impact and reduced cardiovascular risk. Unfiltered coffee methods allow cholesterol-raising compounds to remain in the cup.
For patients concerned about cholesterol, simple changes such as switching brewing methods can support lipid management without eliminating coffee.
As research continues, coffee remains a complex beverage with both benefits and risks. Understanding preparation methods allows consumers and clinicians to make informed choices that support long-term health.
Coffee does affect cholesterol, but the impact depends mainly on brew method. Unfiltered coffee methods, such as French press, Turkish, and boiled coffee, allow compounds that raise LDL cholesterol to pass into the drink. Filtered coffee removes these compounds and is associated with better heart outcomes.
For most people, filtered coffee consumed in moderation is the healthiest choice. Those with elevated cholesterol should pay close attention to brewing method as part of a comprehensive approach to cardiovascular health.
Coffee is not just about taste or routine. It is also about chemistry and preparation. Small changes in coffee preparation can lead to meaningful differences in long-term health outcomes.
Employee wellness directly impacts productivity and long-term success; understanding the science behind daily habits is essential. Contact Metis Consulting Services today for more info: Hello@metisconsultingservices.com