Navigating Intense Drug Market Pressures
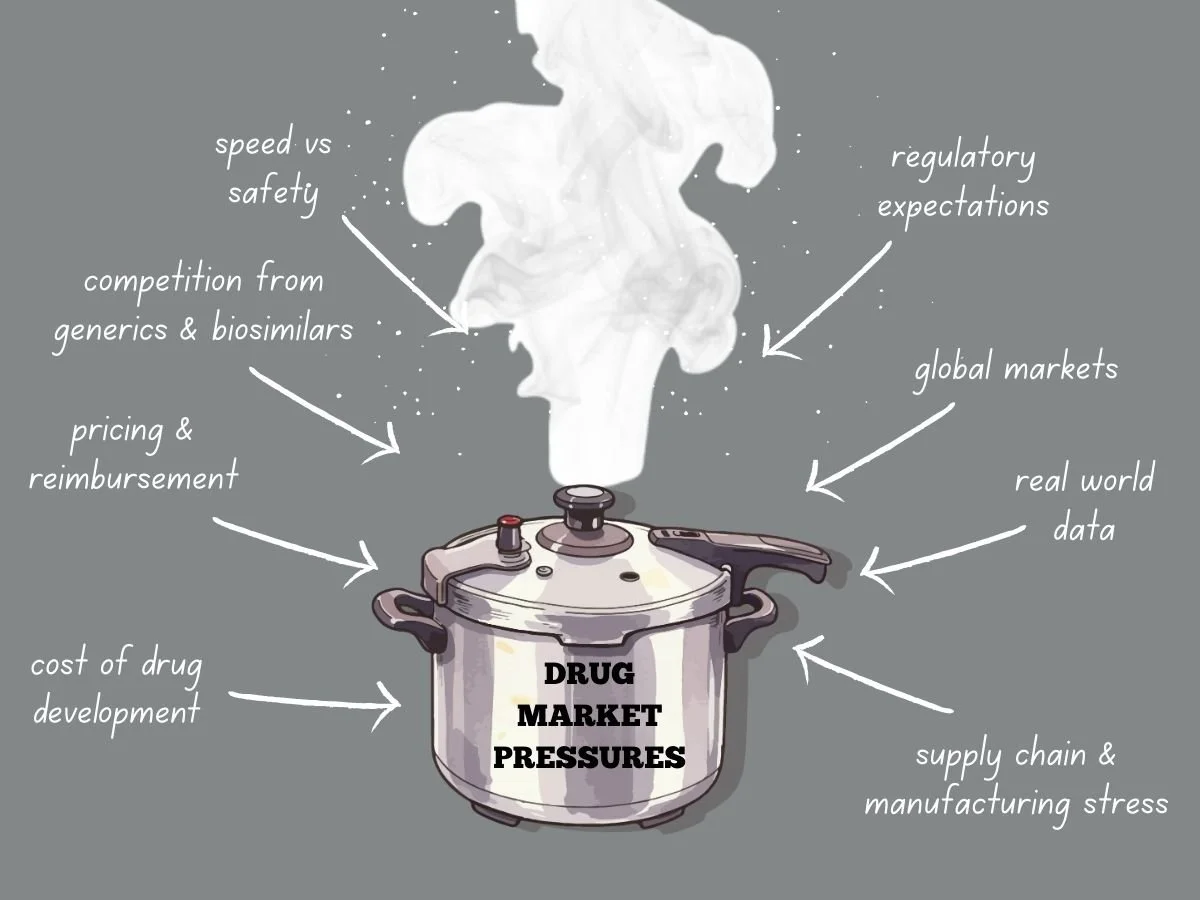
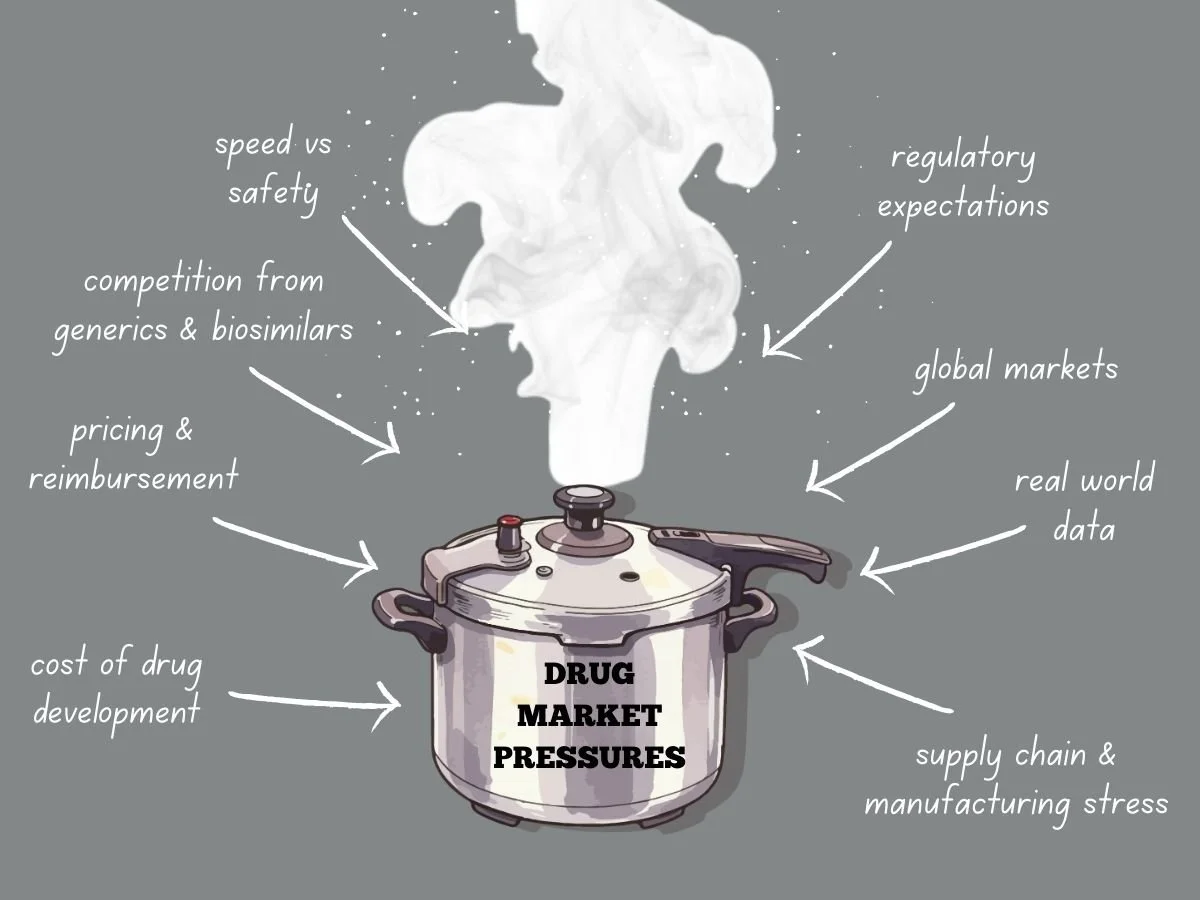
Today’s global drug market faces more pressure than ever before. Pharmaceutical companies deal with higher development costs, tougher regulations, stricter pricing, and more competition from generics and biosimilars. Meanwhile, patients, payers, and governments want quicker access to safe and effective treatments. These factors are changing how companies plan, develop, launch, and manage products over time.
To succeed, companies need to know what causes market pressure and how it shapes their decisions. They also have to adjust their strategies while keeping quality, safety, and compliance intact.
This week in the Guardrail, we explore the intensifying economic and regulatory forces reshaping the global pharmaceutical landscape. We analyze the ways industry leaders can maintain compliance and quality while navigating the high-stakes pressures of modern drug development.
By Michael Bronfman for Metis Consulting Services
January 19, 2026
Today’s global drug market faces more pressure than ever before. Pharmaceutical companies deal with higher development costs, tougher regulations, stricter pricing, and more competition from generics and biosimilars. Meanwhile, patients, payers, and governments want quicker access to safe and effective treatments. These factors are changing how companies plan, develop, launch, and manage products over time.
To succeed, companies need to know what causes market pressure and how it shapes their decisions. They also have to adjust their strategies while keeping quality, safety, and compliance intact.
The Cost Reality of Drug Development
Developing new drugs is both costly and risky. It can take billions of dollars to bring a new medicine to market, especially when you include failed attempts. Clinical trials last for years, regulatory submissions need a lot of data, and manufacturing must meet rigorous quality standards.
As development costs go up, so does market pressure. Investors want to see returns, so leaders have to focus on programs most likely to succeed. This means making tough choices about which therapies to continue and which to pause or stop.
Pressure grows when competitors work on similar products. If a company is second to market, it can lose pricing power and market share. This pushes companies to find ways to develop products faster while still following the rules.
Pricing and Reimbursement Challenges
Pricing pressure is a major challenge in today’s drug market. Governments and private payers are resisting high launch prices, and value-based pricing is becoming more common. Companies now have to clearly show clinical benefits, real-world results, and economic value.
In the United States, pricing scrutiny continues to grow through policy changes and public debate. Programs like Medicare negotiation place additional pressure on manufacturers to justify pricing decisions. The NIH National Library of Medicine is a good resource for more information at https://pmc.ncbi.nlm.nih.gov/articles/PMC11129567/
In Europe, pricing and reimbursement decisions are often made at the country level. Health technology assessments play a significant role in determining whether a product will be reimbursed and at what price. The European Medicines Agency provides regulatory approval, but market access depends on additional reviews. https://globalpricing.com/pricing-and-reimbursement-trends-in-europe-current-landscape-and-implications/
Because of these pressures, companies can not wait until after approval to plan for market access. They need to start early, thinking about evidence, comparators, and patient groups.
Competition from Generics and Biosimilars
Patent expiration is still a major source of market pressure. Once exclusivity ends, generics and biosimilars can quickly cut into revenue. Sometimes, prices fall by over 80 percent in the first year.
Companies have to plan their product life cycles early. They might consider new formulations, more uses, or combination products. Each choice needs careful regulatory planning and strong supporting data.
The US Food and Drug Administration provides guidance on generics and biosimilars, including approval pathways and exclusivity considerations at https://www.fda.gov/drugs/development-approval-process-drugs/how-drugs-are-developed-and-approved.
Companies that wait too long to plan for the loss of exclusivity often have trouble protecting their product’s value when competitors arrive.
Speed Versus Safety
Market pressure often makes teams move faster. Quicker development can help patients get treatments sooner and improve a company’s position. But moving too fast without proper controls can lead to big risks.
Rushing trials can lead to poor study design or trouble enrolling patients. If data is incomplete, it can cause regulatory delays or extra work after approval. Cutting corners in manufacturing can cause quality problems and require more inspections.
To balance speed and safety, companies need strong oversight. Clear decision rules, teamwork across departments, and early talks with regulators are key. Programs that focus on quality from the beginning handle market pressure better.
Regulatory Expectations Do Not Ease Under Pressure
One common misconception is that regulators may be more flexible when market needs are urgent. While there are faster review programs, the standards for safety, effectiveness, and quality do not change for the Fast Track and Breakthrough Therapy designation. These programs aim to speed access while maintaining standards.
The FDA provides guidance:
But it can be tricky to navigate these expectations.
Using these pathways successfully requires careful planning and ongoing communication with regulators. Market pressure is not a good reason for weak data or incomplete submissions. This adds another layer of pressure. Regulatory requirements vary by region. Clinical trial designs must support multiple agencies. Manufacturing and labeling must meet diverse standards.
If regions are not aligned, it can cause delays and extra costs. For example, a trial designed just for the US might not work in Europe or Asia. Aligning global strategy early helps avoid these problems.
The International Council for Harmonisation plays a key role in aligning technical requirements across regions. Information on these guidelines is available at https://www.ich.org/page/search-index-ich-guidelines.
Understanding and applyLearning and using these guidelines early helps companies handle global market pressure better.
Global Markets Add Complexity
Many products are developed for global markets. This adds another layer of pressure. Regulatory requirements vary by region. Clinical trial designs must support multiple agencies. Manufacturing and labeling must meet diverse standards.
Misalignment between regions can lead to delays and added costs. For example, a trial designed only to meet US requirements may fall short in Europe or Asia. Early global strategy alignment helps reduce this risk.
The International Council for Harmonisation plays a key role in aligning technical requirements across regions. Information on guidelines is available at https://www.ich.org/page/search-index-ich-guidelines
Understanding and applying these guidelines early helps companies manage global market pressure more effectively.
Manufacturing Stress
Market pressure continues after approval. Manufacturing and supply chains have their own problems. It’s hard to predict demand, especially for new products. Shortages, global issues, and quality problems can all disrupt supply. Manufacturers to maintain control and continuity of supply. Inspections focus on data integrity, process validation, and change management. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations
When under a lot of pressure, companies might try to stretch their capacity or put off investments. These choices often increase risk and can lead to regulatory problems.
The Role of Real World Evidence
As pricing and access decisions rely more on data, real-world evidence is becoming more important. Payers and regulators want to know how products work outside of controlled trials.
To collect good real-world data, companies need planning, the right systems, and oversight. They also have to meet regulatory standards for data reliability and privacy.
https://www.fda.gov/science-research
Companies that build these capabilities early are better able to handle market pressure and keep their products valuable over time.
Organizational Alignment Under Pressure
Market pressure often reveals weak spots in how organizations are set up. Gaps between clinical, regulatory, quality, and commercial teams can slow decisions. Conflicting goals can also cause teams to lose focus.
Successful organizations set shared goals and maintain open communication. They invest in training and clear processes. Leaders make it clear that compliance and quality always come first, even when deadlines are tight.
This kind of culture is essential when companies face inspections, audits, or public attention.
Looking Ahead
Drug market pressures are not going away. If anything, they will continue to intensify. Companies that treat pressure as a reason to cut corners will face setbacks. Those who use pressure as a driver for more thoughtful planning, stronger execution, and earlier collaboration will be better positioned to succeed.
Navigating this environment requires discipline, foresight, and respect for regulatory expectations. It also requires a clear focus on patients, who remain the ultimate reason these products exist.
The path from laboratory to patient has never been more complex, but you don't have to navigate these regulatory and economic hurdles alone. Contact Metis Consulting Services today. Learn how our strategic oversight and industry expertise can help your organization transform market pressure into a sustainable competitive advantage.
Understanding the Significance of CRLs Being Released: Beyond the Regulatory Language
The FDA's Complete Response Letter (CRL)-- few documents hold as much weight in the complex and often opaque world of pharmaceutical development, as the CRL. Metis Consulting can help navigate them, learn more at our blog, The Guard Rail.
Written by Michael Bronfman, July 28, 2025
This week in The Guard Rail, we at Metis are looking at a hot topic for our industry. Michael Bronfman tackles a hidden power in the pharmaceutical and medical device manufacturing world: the FDA's Complete Response Letters (CRLs). These are not just dry documents. The contents have traditionally been kept secret, known only to the receiving company. However, that secrecy might now be coming into the open. Why? Because a CRL can instantly derail a company's future, send stock prices plummeting, and, most critically, determine if a life-saving treatment ever sees the light of day. Join us as we uncover why these once-confidential letters are at the heart of a tidal wave push for transparency.
The FDA's Complete Response Letter (CRL)-- few documents hold as much weight in the complex and often opaque world of pharmaceutical development, as the CRL. For many outside the industry, the term might sound dry, bureaucratic, or even cryptic. But for drug developers, investors, patients, and clinicians, CRLs are pivotal turning points; letters that can reshape company strategy, impact stock prices overnight, and, most importantly, influence when or even if a new therapy reaches patients.
Historically, the contents of CRLs have often remained confidential, known only to the company receiving them and occasionally, selectively disclosed to the public. Yet the idea of CRLs being more broadly released, whether voluntarily by sponsors or systematically through policy change has gained traction. Why? Let us explore why these letters matter, what they contain, and why making them public can be a significant step forward for science, business, and patient trust.
What exactly is a CRL?
A Complete Response Letter is issued by the U.S. Food and Drug Administration (FDA) when it completes its review of a New Drug Application (NDA) or Biologics License Application (BLA) but decides not to approve it in its current form. Importantly, a CRL does not mean the drug is permanently rejected. Instead, it outlines the deficiencies that prevent approval and often provides guidance on what the sponsor could do to address them.
Deficiencies can include:
Issues with clinical efficacy or safety data (e.g., not enough evidence that the drug works, or safety concerns in certain patient populations)
Manufacturing or quality control shortcomings
Problems with labeling or risk management strategies
Statistical or methodological issues in trial design
For sponsors, receiving a CRL is both a setback and a roadmap. It’s an official document telling them: “Here is what is missing; come back when you have fixed it.”
The FDA's Complete Response Letter (CRL) few documents hold as much weight, in the complex and often opaque world of pharmaceutical development, as the CRL. For many outside the industry, the term might sound dry, bureaucratic, or even cryptic. But for drug developers, investors, patients, and clinicians, CRLs are pivotal turning points; letters that can reshape company strategy, impact stock prices overnight, and, most importantly, influence when or even if a new therapy reaches patients.
Historically, the contents of CRLs have often remained confidential, known only to the company receiving them and occasionally, selectively disclosed to the public. Yet the idea of CRLs being more broadly released — whether voluntarily by sponsors or systematically through policy change — has gained traction. Why? Let's explore why these letters matter, what they contain, and why making them public can be a significant step forward for science, business, and patient trust.
What exactly is a CRL?
A Complete Response Letter is issued by the U.S. Food and Drug Administration (FDA) when it completes its review of a New Drug Application (NDA) or Biologics License Application (BLA) but decides not to approve it in its current form. Note, a CRL does not mean the drug is permanently rejected. Instead, it outlines the deficiencies that prevent approval. Often, the letter will provide guidance on what the sponsor can do to address the issue.
Deficiencies can include:
Issues with clinical efficacy or safety data (e.g., not enough evidence that the drug works, or safety concerns in certain patient populations)
Manufacturing or quality control shortcomings
Problems with labeling or risk management strategies
Statistical or methodological issues in trial design
For sponsors, receiving a CRL can be a setback, but it is also can be a roadmap. It is an official document that says: "Here is what is missing; come back when you have fixed it."
Why are CRLs so important?
CRLs carry enormous significance because they sit at the intersection of science, business, and public health. Consider:
1. Strategic pivot points for companies
A CRL forces a company to decide: Do we invest more time and money to address the FDA's concerns, or do we walk away? Sometimes the deficiencies are minor and easily fixable; at other times, they are so fundamental that continuing to do so makes little sense.
2. Market-moving disclosures
Because the market places great value on new product approvals, the news of a CRL often leads to sharp drops in a company's stock price — especially if the drug was seen as a major pipeline asset.
3. Impact on patients
For patients waiting for new treatment options, CRLs can feel like an unexpected delay. Understanding the nature of the deficiency can help patients and advocates see whether it is a temporary hurdle or a sign of deeper problems.
4. Scientific learning
Each CRL is a detailed FDA critique of a drug's data and the sponsor's responses. While usually kept confidential, if shared, they can become case studies that improve drug development as a whole.
The current situation: Confidential by default
Under U.S. law, CRLs are part of a company's regulatory correspondence and thus are treated as confidential commercial information. Sponsors may choose to disclose the fact that they received a CRL — and often do, given that it's material information for investors — but the actual content is rarely released in full.
Instead, companies often issue press releases summarizing the FDA's concerns. Unfortunately, these summaries can be selective, vague, and overly optimistic:
Selective: emphasizing easily fixable manufacturing issues and omitting more serious efficacy concerns
Vague: using language like "additional analyses requested" without context
Optimistic: framing the CRL as "a minor setback" even if the letter itself is more critical
This practice makes it hard for outside observers — including investors, clinicians, and patient groups — to understand what really happened.
The significance of CRLs being more publicly released
CRLs regularly released in full, could have a profound effect on how new therapies are evaluated, understood, and debated. Here's why:
1. Transparency builds trust
Our industry struggles with perceptions of secrecy. Polished summaries are shared and that is fine but if they are the only data released, it is impossible to know if the sponsor is downplaying serious concerns. Releasing more complete CRLs shows the unfiltered FDA perspective, which can reassure the public that approvals are based on thorough, science-driven review.
2. Better information for stakeholders
Investors could better assess the real risk of resubmission and approval. Clinicians could understand why certain drugs were not approved — whether due to safety concerns in specific populations or inadequate evidence of benefit. Patients and advocacy groups could advocate more effectively if they knew the precise barriers.
3. Industry-wide learning
Drug development is full of repeated mistakes: inadequate trial design, poor endpoint selection, underpowered studies, or manufacturing gaps. Public CRLs can serve as detailed case studies, allowing future sponsors to avoid similar pitfalls.
4. Accountability
Public CRLs help ensure that sponsors fully address the FDA's concerns before resubmitting, rather than trying to sidestep them with minimal new data. They also keep the FDA accountable, making its reasoning transparent and open to scientific debate.
Potential drawbacks and industry concerns
Of course, releasing CRLs is not without controversy. Key concerns include:
1. Proprietary data
CRLs often contain detailed discussion of clinical trial data, manufacturing processes, and commercial plans. Sponsors argue that full disclosure could benefit competitors or harm competitive advantage.
2. Misinterpretation
FDA reviews are technical documents, and taken out of context, statements in a CRL could be misread by the public or sensationalized by the media.
3. Chilling effect on communication
If sponsors know that every word in their submissions could become public, they might be less candid, potentially limiting open dialogue with regulators.
4. Impact on innovation
Some fear that too much transparency could discourage small biotech firms — already operating under tight timelines and budgets — from pursuing high-risk programs.
The evolving conversation
The debate is not purely academic. In recent years:
Some sponsors have voluntarily released CRLs, especially when the market reaction to vague summaries was worse than anticipated.
Regulatory advocates and transparency groups have pushed for routine publication, arguing that CRLs, like European Public Assessment Reports (EPARs), could help demystify the approval process.
The FDA itself has signaled interest in improving transparency, though it is constrained by existing confidentiality laws.
The conversation reflects a broader trend in medicine: moving from "trust us" to "show us." Patients, payers, and clinicians want to see the data and the reasoning behind it, not just the headline.
International context
The U.S. FDA is not alone in grappling with this issue. European regulators, through the EMA, publish relatively detailed assessment reports once a drug is approved, but not if it is rejected. Similarly, Health Canada has taken steps to publish "Summary Basis of Rejection" documents for drugs that are not approved.
These models demonstrate that it is possible to balance transparency with the protection of confidential information, although it requires careful policy design.
A path forward
So, what would be the ideal outcome?
Routine publication of redacted CRLs: Share the FDA's reasoning while redacting truly proprietary data, like detailed manufacturing process steps.
Standardized summaries: Even if full letters aren't released, require sponsors to issue standardized, FDA-reviewed summaries that accurately reflect the deficiencies.
Educational context: Provide plain-language explanations alongside CRLs, so clinicians, patients, and journalists can understand the technical details.
Such steps could bring real benefits without undermining innovation.
Why it matters
At its heart, the significance of CRLs being released is about more than a document. It is about shining light on critical moments in the life of a new therapy: the point where data meets judgment. When companies keep those moments private, the public can only guess at what went wrong. When CRLs are shared, everyone from researchers designing the next trial to patients hoping for a breakthrough can see, learn, and plan accordingly.
Transparency is not a cure-all. It won't eliminate uncertainty, disappointment, or risk. However, in a field where trust is essential and decisions affect both lives and balance sheets, sharing the FDA's reasoning is a powerful way to build confidence, foster learning, and ultimately bring better medicines to the people who need them.
If your organization is grappling with CRLs or needs help avoiding them, please contact us at Metis Consulting Services: Hello@MetisConsultingServices.com.
For more info, see our website www.MetisConsultingServices.com
Navigating FDA Oversight in an Era of Advanced Digital Tools
By Michael Bronfman, July 14, 2025
The pharmaceutical industry is undergoing a transformation. Across the drug development lifecycle, from early discovery through clinical trials and into postmarket monitoring, companies increasingly rely on sophisticated digital tools. These tools analyze complex data, personalize treatments, and speed up development. However, as these digital systems begin to inform decisions traditionally in the hands of clinicians or regulators, the U.S. Food and Drug Administration (FDA) is adapting its regulatory framework accordingly.
For biotech professionals, this means that digital tools are no longer optional supports, they are deeply intertwined with product strategy and regulatory planning. This post explores how digital technologies are reshaping the regulatory landscape, what it means for pharma companies, and the practical steps organizations must take to thrive.
1. Digital Innovation in Pharma: Opportunity and Responsibility
The industry is leveraging digital capabilities in areas such as:
Target identification and compound screening: using pattern recognition systems to highlight promising molecule targets.
Clinical trial efficiency: tools that help select study sites, recruit patients, or monitor data in real time.
Image analysis in diagnostics: supporting clinical insights through automated interpretation of scans or pathology slides.
Postmarket surveillance: identifying safety signals and performance trends from real-world data.
Patient engagement platforms: improving compliance, remote monitoring, and decentralized trial models.
These tools can significantly reduce time and cost, improve decision-making, support personalized approaches, and with increased impact comes increased scrutiny.
Regulators now expect the same rigor, transparency, and oversight for digital tools as for manual tools.
2. The FDA’s Strategic Response
The FDA has long recognized the growing role of technology in clinical care and has been refining its regulatory oversight:
SaMD Framework (Software as a Medical Device): Software that diagnoses, treats, or manages patient care falls under medical device regulations. The FDA applies standards for safety, effectiveness, and Quality.
Proposal for Iterative Updates (2019): The agency introduced methods for handling software that adapts post-approval, suggesting that plans be in place to anticipate upgrades.
Action Plan (2021):
This plan emphasized:
1. Clear documentation of tool design and data use
2. Risk and bias evaluation
3. Transparency and explainability
4. Postmarket monitoring
5. Collaboration with global regulators and external experts
Digital Health Advisory Committee (established 2023): Brings together external leaders to advise the FDA on emerging digital health trends, including data platforms and analysis tools.
Taken together, these efforts show the FDA is no longer reactive—it’s taking steps to guide the shift toward intelligent, data-driven healthcare responsibly.
3. Why This Matters to Pharma Companies
When digital tools are used to inform diagnosis, treatment, or clinical decisions, they are treated as regulated medical products, not simple IT solutions. This has several consequences:
Raised Standards for Evidence and Validation:
Digital tools must now deliver clear, reproducible performance:
Auditable data lineage: where data comes from, how it was processed
Testing in real-world settings and across diverse patient groups
Bias assessments to ensure performance isn’t limited to specific subpopulations
Explainable outputs so clinicians and patients trust the insights
These developing supportive tools in trials must meet these requirements.
Managing Tools that Evolve Over Time
Unlike a tablet with a fixed formula, software can be updated. The FDA expects companies planning to:
Define what changes are permissible
Assess the impact and validate updates
Communicate effectively with regulators and end users
This is often captured in a Predetermined Change Control Plan (PCCP). Whether it’s a predictive model or diagnostic classifier, understanding the change process and its controls becomes essential.
Implications for Clinical Trials
When digital tools:
Support trial operations (by speeding recruitment or monitoring risk) they must be shown not to skew results or introduce bias.
Serve as the trial’s intervention (e.g., diagnostics or decision support systems) they need their own efficacy and safety data, potentially requiring standalone validation or randomized comparisons.
This dual role calls for early regulatory planning and deep engagement with trial design teams.
Increased Focus on Post-Market Oversight
The FDA now expects:
Ongoing monitoring after product launch
Collection of real-world performance data
Alert systems for declining tool performance or unexpected failures
Protocols for updating the tool and notifying regulators or users.
This mirrors pharmacovigilance demands and supports long-term patient safety.
4. What Pharma Executives Should Watch
In the coming months and years, several developments will shape digital tool regulation:
Final Edited Guidance on Adaptive Tools
We can expect finalized positions covering:
Permissible software updates
Required audit trails
Performance metrics and thresholds
Monitoring and reporting protocols
Aligning technology roadmaps to these expected updates will smooth regulatory
Reviews.
Global Harmonization Efforts
Agencies such as EMA (Europe) and IMDRF (international) are converging on:
Data governance
Model transparency
Security and privacy safeguards
Pharma firms operating cross-border must design systems that comply across jurisdictions.
Evolving Quality Standards
Expect new additions to quality standards, including Good Machine Learning Practices
(GMLP) and guidance on digital quality systems, covering:
Metadata and dataset versioning
Traceability of analysis and results
Risk management for software failure
Early adoption helps avoid later compliance issues.
Liability and Responsibility Issues
As intelligent tools play bigger roles, questions arise:
Who is responsible if a tool provides flawed guidance?
What disclaimers or training must accompany tools?
How are clinicians involved in oversight?
Proactive definition of roles, responsibilities, and risk management processes now can help minimize legal exposure.
Prioritizing Trust and Interpretability
Stakeholders increasingly demand:
Intuitive, explainable interfaces
Clear output and user instructions
Evidence that supports clinical decision-making
Transparent tools are more trusted—and more likely to sail through regulatory evaluation.
5. Action Plan for Pharma Leaders
To stay ahead, companies should take these definitive steps:
Form a Cross-Functional Digital Oversight Committee
Include regulatory, clinical, IT, data science, legal, and quality assurance leaders from the start.
Classify All Digital Initiatives Early
Identify which tools may require regulatory filings, versus those that support internal operations.
Create Clear Documentation Standards
Maintain logs of:
Data sources and preprocessing steps
Model tests and performance evaluations
Change histories and validation results
Incident logs and monitoring updates
Engage Regulators Early
Use the FDA’s QSubmission (presubmission) process to preview plans, especially for trailblazing tools.
Build Post-Deployment Infrastructure
Plan upfront for:
Routine performance audits
Data pipelines for real-world monitoring
Reporting processes for updates or safety concerns
Train Users and Maintain Accountability
Educate clinicians and trial sites on:
The tool’s purpose and scope
How outputs should and shouldn’t be used
When to escalate concerns or deviations
Include user accountability protocols to reinforce oversight.
6. Case Examples: Learning from the Field
While specific details vary, high-level examples illustrate these principles:
Digital diagnostics used in trial site selection:
Validated on diverse patient data, with ongoing monitoring to ensure fair representation.
Automated image analysis used for tumor response:
Incorporated early feedback from the FDA but included plans for updates, accuracy validations, and clarity documentation.
Remote patient monitoring device:
Treated as a regulated device—complete with device history record, software verification benchmarks, and firmware update protocols.
These mature implementations underscore the necessity of structured design, planning, and oversight through the entire tool lifecycle.
Aligning Digital Ambition with Regulatory Expectations
Pharmaceutical companies today are stepping up digital innovation, fueled by data advances and software capabilities, and the balance of opportunity and risk now includes a regulatory dimension: advanced tools are no longer optional, they are regulated.
To lead responsibly:
Treat digital tools as core products
Build in line with regulatory principles
Document everything comprehensively
Continue oversight through deployment and updates
Embracing this approach protects compliance and fosters market adoption and trust.
The Path Forward
Pharma’s digital transformation is accelerating. When executed with foresight and regulatory alignment, digital tools can enhance safety, speed, and efficacy. They must be built with process, governance, and accountability at their core. By mapping development to regulatory frameworks, designing for continuous oversight, and integrating quality systems from the start, companies can harness innovation while meeting the expectations of regulators, clinicians, and patients.
The coming years will not be about whether your organization uses digital tools, but rather how responsibly, transparently, and effectively those tools are designed and managed. Those who plan accordingly will set the standard, and those who hesitate risk falling behind.
If you are looking for guidance and advice on how to take your organization to the forefront of this technology, and how to embrace it. Email us at Hello@metisconsultingservices.com or check out our website www.metisconsultingservices.com
Our experts will help you navigate the future of Pharmaceutical and Medical Device manufacturing.