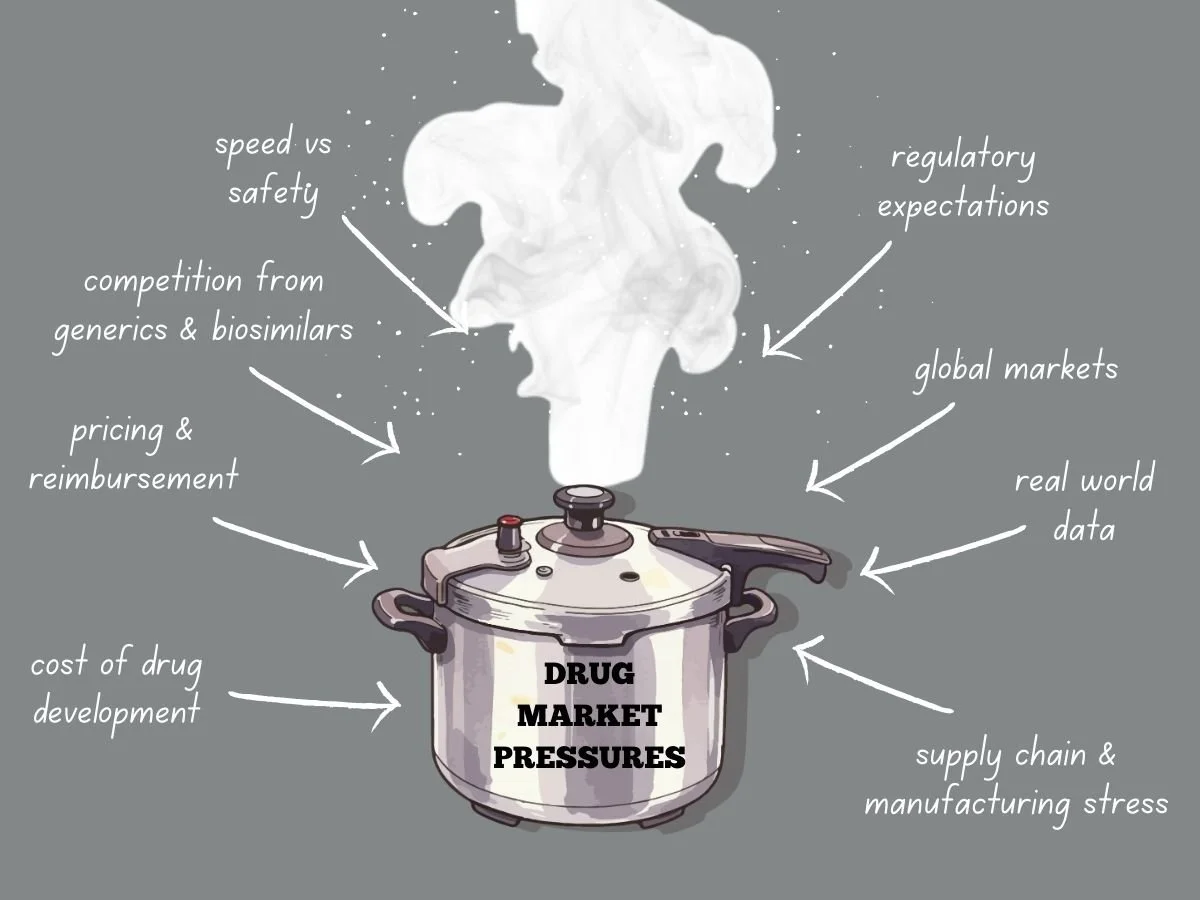
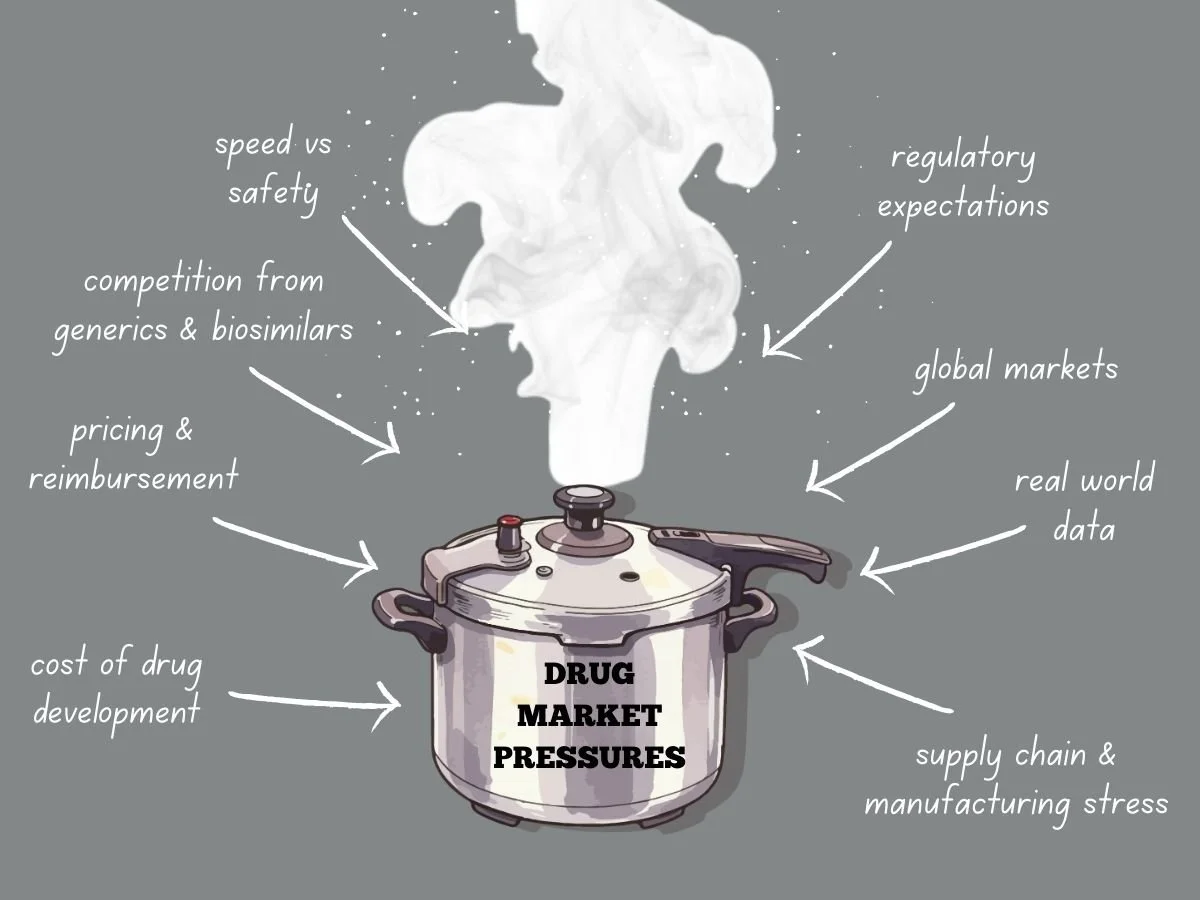
Navigating Intense Drug Market Pressures
Today’s global drug market faces more pressure than ever before. Pharmaceutical companies deal with higher development costs, tougher regulations, stricter pricing, and more competition from generics and biosimilars. Meanwhile, patients, payers, and governments want quicker access to safe and effective treatments. These factors are changing how companies plan, develop, launch, and manage products over time.
To succeed, companies need to know what causes market pressure and how it shapes their decisions. They also have to adjust their strategies while keeping quality, safety, and compliance intact.
This week in the Guardrail, we explore the intensifying economic and regulatory forces reshaping the global pharmaceutical landscape. We analyze the ways industry leaders can maintain compliance and quality while navigating the high-stakes pressures of modern drug development.
By Michael Bronfman for Metis Consulting Services
January 19, 2026
Today’s global drug market faces more pressure than ever before. Pharmaceutical companies deal with higher development costs, tougher regulations, stricter pricing, and more competition from generics and biosimilars. Meanwhile, patients, payers, and governments want quicker access to safe and effective treatments. These factors are changing how companies plan, develop, launch, and manage products over time.
To succeed, companies need to know what causes market pressure and how it shapes their decisions. They also have to adjust their strategies while keeping quality, safety, and compliance intact.
The Cost Reality of Drug Development
Developing new drugs is both costly and risky. It can take billions of dollars to bring a new medicine to market, especially when you include failed attempts. Clinical trials last for years, regulatory submissions need a lot of data, and manufacturing must meet rigorous quality standards.
As development costs go up, so does market pressure. Investors want to see returns, so leaders have to focus on programs most likely to succeed. This means making tough choices about which therapies to continue and which to pause or stop.
Pressure grows when competitors work on similar products. If a company is second to market, it can lose pricing power and market share. This pushes companies to find ways to develop products faster while still following the rules.
Pricing and Reimbursement Challenges
Pricing pressure is a major challenge in today’s drug market. Governments and private payers are resisting high launch prices, and value-based pricing is becoming more common. Companies now have to clearly show clinical benefits, real-world results, and economic value.
In the United States, pricing scrutiny continues to grow through policy changes and public debate. Programs like Medicare negotiation place additional pressure on manufacturers to justify pricing decisions. The NIH National Library of Medicine is a good resource for more information at https://pmc.ncbi.nlm.nih.gov/articles/PMC11129567/
In Europe, pricing and reimbursement decisions are often made at the country level. Health technology assessments play a significant role in determining whether a product will be reimbursed and at what price. The European Medicines Agency provides regulatory approval, but market access depends on additional reviews. https://globalpricing.com/pricing-and-reimbursement-trends-in-europe-current-landscape-and-implications/
Because of these pressures, companies can not wait until after approval to plan for market access. They need to start early, thinking about evidence, comparators, and patient groups.
Competition from Generics and Biosimilars
Patent expiration is still a major source of market pressure. Once exclusivity ends, generics and biosimilars can quickly cut into revenue. Sometimes, prices fall by over 80 percent in the first year.
Companies have to plan their product life cycles early. They might consider new formulations, more uses, or combination products. Each choice needs careful regulatory planning and strong supporting data.
The US Food and Drug Administration provides guidance on generics and biosimilars, including approval pathways and exclusivity considerations at https://www.fda.gov/drugs/development-approval-process-drugs/how-drugs-are-developed-and-approved.
Companies that wait too long to plan for the loss of exclusivity often have trouble protecting their product’s value when competitors arrive.
Speed Versus Safety
Market pressure often makes teams move faster. Quicker development can help patients get treatments sooner and improve a company’s position. But moving too fast without proper controls can lead to big risks.
Rushing trials can lead to poor study design or trouble enrolling patients. If data is incomplete, it can cause regulatory delays or extra work after approval. Cutting corners in manufacturing can cause quality problems and require more inspections.
To balance speed and safety, companies need strong oversight. Clear decision rules, teamwork across departments, and early talks with regulators are key. Programs that focus on quality from the beginning handle market pressure better.
Regulatory Expectations Do Not Ease Under Pressure
One common misconception is that regulators may be more flexible when market needs are urgent. While there are faster review programs, the standards for safety, effectiveness, and quality do not change for the Fast Track and Breakthrough Therapy designation. These programs aim to speed access while maintaining standards.
The FDA provides guidance:
But it can be tricky to navigate these expectations.
Using these pathways successfully requires careful planning and ongoing communication with regulators. Market pressure is not a good reason for weak data or incomplete submissions. This adds another layer of pressure. Regulatory requirements vary by region. Clinical trial designs must support multiple agencies. Manufacturing and labeling must meet diverse standards.
If regions are not aligned, it can cause delays and extra costs. For example, a trial designed just for the US might not work in Europe or Asia. Aligning global strategy early helps avoid these problems.
The International Council for Harmonisation plays a key role in aligning technical requirements across regions. Information on these guidelines is available at https://www.ich.org/page/search-index-ich-guidelines.
Understanding and applyLearning and using these guidelines early helps companies handle global market pressure better.
Global Markets Add Complexity
Many products are developed for global markets. This adds another layer of pressure. Regulatory requirements vary by region. Clinical trial designs must support multiple agencies. Manufacturing and labeling must meet diverse standards.
Misalignment between regions can lead to delays and added costs. For example, a trial designed only to meet US requirements may fall short in Europe or Asia. Early global strategy alignment helps reduce this risk.
The International Council for Harmonisation plays a key role in aligning technical requirements across regions. Information on guidelines is available at https://www.ich.org/page/search-index-ich-guidelines
Understanding and applying these guidelines early helps companies manage global market pressure more effectively.
Manufacturing Stress
Market pressure continues after approval. Manufacturing and supply chains have their own problems. It’s hard to predict demand, especially for new products. Shortages, global issues, and quality problems can all disrupt supply. Manufacturers to maintain control and continuity of supply. Inspections focus on data integrity, process validation, and change management. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations
When under a lot of pressure, companies might try to stretch their capacity or put off investments. These choices often increase risk and can lead to regulatory problems.
The Role of Real World Evidence
As pricing and access decisions rely more on data, real-world evidence is becoming more important. Payers and regulators want to know how products work outside of controlled trials.
To collect good real-world data, companies need planning, the right systems, and oversight. They also have to meet regulatory standards for data reliability and privacy.
https://www.fda.gov/science-research
Companies that build these capabilities early are better able to handle market pressure and keep their products valuable over time.
Organizational Alignment Under Pressure
Market pressure often reveals weak spots in how organizations are set up. Gaps between clinical, regulatory, quality, and commercial teams can slow decisions. Conflicting goals can also cause teams to lose focus.
Successful organizations set shared goals and maintain open communication. They invest in training and clear processes. Leaders make it clear that compliance and quality always come first, even when deadlines are tight.
This kind of culture is essential when companies face inspections, audits, or public attention.
Looking Ahead
Drug market pressures are not going away. If anything, they will continue to intensify. Companies that treat pressure as a reason to cut corners will face setbacks. Those who use pressure as a driver for more thoughtful planning, stronger execution, and earlier collaboration will be better positioned to succeed.
Navigating this environment requires discipline, foresight, and respect for regulatory expectations. It also requires a clear focus on patients, who remain the ultimate reason these products exist.
The path from laboratory to patient has never been more complex, but you don't have to navigate these regulatory and economic hurdles alone. Contact Metis Consulting Services today. Learn how our strategic oversight and industry expertise can help your organization transform market pressure into a sustainable competitive advantage.
Why the Lowest Bid in Pharma Services Is Often Too Low
At the Guard Rail this week, we look at how a smart, forward-thinking approach to choosing the right consultants, managing risks, and making strategic decisions can lead to new opportunities and build lasting stability for your business
For Metis Consulting Services
By Michael Bronfman
September 29, 2025
At the Guard Rail this week, we look at how a smart, forward-thinking approach to choosing the right consultants, mitigating risks, and making strategic decisions can lead to new opportunities and build lasting stability for your business.
The pharmaceutical industry is built on quality, safety, and results. When pharmaceutical companies select partners for clinical data management, audits, pharmacovigilance, medical consulting, or healthcare IT consulting, they face a recurring choice. Should they go with the lowest bid? At first, the lowest price may seem like a smart business move. However, in the world of pharma services, the lowest bid is usually low for a reason.
Today, we examine why cutting costs can harm a pharma company, its patients, and its long-term growth. Additionally, we will discuss the importance of quality in pharmaceutical drug development, medical device consulting, and pharmaceutical consulting. The goal is to explain why careful investment in quality matters more than saving a small amount of money at the start.
The Hidden Risks Behind the Lowest Bid in the Pharmaceutical Industry
In many industries, you can take the lowest bid and get acceptable results. In the pharmaceutical industry, that risk is far greater. Drug companies are responsible for the lives of patients. A mistake in clinical data management or pharmacovigilance can lead to unsafe products reaching the market.
When a pharma company accepts the lowest bid, it may end up with weak quality control, untrained staff, or poor systems. These weaknesses may not be apparent immediately. However, they often create hidden risks that damage safety and reputation later.
Clinical Data Management Requires More
Clinical data management is one of the most critical steps in pharmaceutical drug development. It ensures that trial data is accurate, clean, and reliable. Cheap services often lack the necessary technology and trained staff to review data carefully.
Poor quality in clinical data management can delay trials, create errors, and even force regulators to reject study results. When this happens, a pharma company loses more money than it saved. Quality data management is an investment, not a place to cut costs.
Audits in Pharma Consulting: Why the Lowest Bid Fails
Audits are central to pharmaceutical consulting and pharma industry Compliance. Audits verify that a pharmaceutical company is adhering to regulations and ethical standards.
When a pharma consulting firm offers the lowest price, it often reduces the time spent on each review. This can mean important details are missed. In the service pharma industry, weak audits lead to compliance failures, fines, and damaged trust with regulators.
In the long run, saving money on audits by choosing the lowest bid costs far more in penalties and delays. Poor quality audits may also lead to poor outcomes of regulatory inspections. If issues are not identified by oversight and audits, the chances are increased that they will be identified by inspectors.
Pharmacovigilance and the Cost of Cutting Corners
Pharmacovigilance is the practice of monitoring the safety of pharmaceutical drugs once they are on the market. This service protects patients and ensures long-term trust in a pharma company.
Low-cost providers may not have the staff or systems to track side effects globally. They may also fail to meet international safety reporting timelines. For drug companies, weak pharmacovigilance can lead to recalls, lawsuits, and loss of reputation.
The truth is clear. Strong pharmacovigilance costs money. Cutting corners in this area is both ethically and financially dangerous.
Why Superior Pharmaceutical and Medical Consultants Are Worth the Investment
Medical consulting and pharma consulting help guide companies through complex clinical, scientific, and regulatory challenges. A pharma company may turn to consulting for advice on medical devices, market access, or pharmaceutical drug strategy.
If the lowest bid is chosen, consultants may lack the knowledge and experience needed to solve real problems. Inexperienced consultants may offer advice that appears sound on paper but ultimately fails in practice.
In consulting healthcare IT and IT consulting for healthcare, a poor bid can mean weak systems, data breaches, and wasted resources. For pharma companies, the lowest price in consulting usually means the lowest value.
Healthcare IT Consulting: The Price of Poor Systems
A good consultant in IT for healthcare supports the secure management of sensitive medical data. Pharma industry leaders rely on technology for research, regulatory submissions, and global reporting.
A low-cost provider may use outdated systems or fail to protect against cyber threats. This puts both patient safety and company data at risk. Security breaches in pharma services are not only costly but also deeply damaging to trust.
Investing in reliable IT systems is critical for the future of the pharmaceutical industry.
Ethical AI in Pharma Consulting: You Get What You Pay For
Today, ethical AI, ethics in AI, and AI and ethics are growing topics in the pharmaceutcal industry. From drug discovery to pharmacovigilance, AI tools support research and safety.
When a pharmaceutical company chooses the lowest bid for AI services, it may end up with systems that disregard ethics and AI guidelines. Cheap services may not follow strong ethics for AI or established AI ethical standards.
This can create bias in trials, unsafe predictions, or regulatory problems. For companies working with AI and ethics, the lowest bid can threaten both science and trust.
Pharma Services Depend on Quality, Not Just Cost
Pharmaceutical services encompass a wide range of areas, including clinical data management, audits, pharmacovigilance, and consulting on medical devices. In each case, the lesson is the same. The lowest bid is not the best choice.
A strong pharma consult may cost more upfront, but the value is in safe products, successful audits, and trusted data. Pharma consulting should never be a race to the bottom.
The Long-Term Cost of Short-Term Savings
Every pharmaceutical company must manage its budget. However, short-term savings often create long-term costs. When a drug company chooses a low bid, it risks poor data, failed audits, unsafe drugs, or weak systems.
The result is delays, fines, recalls, and lost trust. Patients, regulators, and partners expect the highest standards in the pharmaceutical industry. A company that cuts corners with the lowest bid sends the wrong signal.
Why Consulting in Healthcare IT and Medical Devices Demands Quality
Consulting IT for pharmaceutical and medical device manufacturing requires advanced knowledge and proven systems. Mistakes in these areas can lead to failed regulatory approvals or harm patient safety.
The lowest bid often means rushed projects, poor training, or incomplete testing. These risks outweigh any savings. For pharmaceutical services, investing in quality is always more cost-effective than cleaning up after a failed project.
The Role of Ethics in Pharma Industry Services
The pharma industry has a duty to protect patients and follow ethical practices. This includes pharmacovigilance, clinical data management, audits, and ethical AI systems.
Cutting costs by picking the lowest bid often leads to missed ethical standards. Strong ethics require time, training, and investment. In areas such as ethics in AI, ethics and AI, and ethics for AI, the lowest price may ignore important safeguards. Trust in the pharmaceutical industry depends on more than cost. It depends on integrity.
In Pharma, You Truly Get What You Pay For
The message is simple but vital. In the pharmaceutical industry, the lowest bid is usually low for a reason. Every pharma company that values patient safety, strong compliance, and long-term success must see the danger of cutting costs.
Pharma consulting, healthcare IT consulting, pharmacovigilance, clinical data management, and medical consulting all require quality over price. The risks of poor service in these areas are too high.
In a field where lives depend on results, you always get what you pay for. Choosing quality over the lowest bid is the only path to lasting trust and success in the pharma industry.
Don't let a focus on short-term savings lead to long-term pain. The initial decision to invest in a superior solution minimizes future risk and the expensive necessity of fixing mistakes down the line. To learn more about how a forward-thinking approach to strategic foresight can save you from costly repairs, contact Metis Consulting Services today. For more details, visit our website at metisconsultingservices.com or email us at hello@metisconsultingservices.com. We're here to help you turn your potential into profit.
The Advantages of Bringing Pharmaceutical CMOs Back to the United States
Bringing CMOs back to the United States
For Metis Consulting Services, Inc.
By Michael Bronfman
August 11, 2025
This week in the "Guard Rail," we at Metis are exploring "Reshoring" of CMOs. We can't afford to settle for anything less than a fortified, domestic, and regional pharmaceutical industry. For decades, the lure of international manufacturing offered a path of lower costs, but this road has proven to be full of potholes.
The Benefits of Bringing Pharmaceutical CMOs Back to the United States (Reshoring)
The pharmaceutical industry plays a central and critical role in public health. Every stage in the drug development and manufacturing process impacts the final quality and safety of medicines. Contract Manufacturing Organizations, known as CMOs, are third-party organizations that manufacture drugs for pharmaceutical firms. These organizations handle activities ranging from producing active pharmaceutical ingredients (API) to packaging and labeling.
Over the past several decades, a large number of pharmaceutical manufacturers have moved overseas. Let's talk about why this is happening: cost savings, reduced labor expenses, and relaxed regulatory environments often tempt companies to China and India.
There have been growing discussions lately about the benefits of bringing pharmaceutical CMOs back to the United States. The term for this movement is "reshoring." The trend to shift overseas has come with a set of challenges and risks that directly impact quality, safety, and national security. Although reshoring requires investment of all kinds, including time and workforce development, among others, it also brings a wide range of returns on those investments. These advantages include improved supply chain resilience, increased product quality, strengthened national security, job creation, and a reduction in reliance on foreign manufacturing. And isn't that what we all want?
Here, we will take a bigger look at how reshoring CMOs to the United States offers long-term benefits to both the pharmaceutical industry and the public.
Improved Supply Chain Reliability
Pharmaceutical manufacturing operates most effectively with a stable supply chain. Delays, shortages, and disruptions have serious consequences for patients' access to the drugs they need. The complexity of global supply chains is in itself a challenge that creates multiple points of vulnerability. Drugs may pass through several countries before reaching their final destination. Disruption along this path, at any point, can lead to delays or stockouts. The long, complex chain is vulnerable to myriad forms of delay, including political tensions, natural disasters, or transportation failures.
By relocating CMOs to the United States, pharmaceutical companies can reduce the number of steps involved in the supply chains. As a result, we would expect faster delivery of finished products and improved response times during public health emergencies. A domestic manufacturing base allows for greater control over production scheduling and inventory management.
During the COVID-19 pandemic, global supply chain disruptions exposed the risks of overdependence on foreign manufacturing. Not just for us here in the US, but globally. Shortages of essential medications and active pharmaceutical ingredients were rampant. A more localized supply chain could help prevent similar problems in the future.
Enhanced Quality Control and Regulatory Oversight
The United States Food and Drug Administration enforces strict regulatory standards. The manufacturers must follow detailed guidelines to ensure safety, consistency, and efficacy. When pharmaceutical companies outsource production to overseas CMOs, consistent quality and quality oversight are more challenging. Regulatory agencies often lack the same reach and oversight capabilities in other countries.
If their CMOs are located back here in the United States, companies gain better access to real-time oversight, audits, inspections, and monitoring. Regulatory compliance is easier to enforce, and deviations from quality standards can be addressed more quickly. This results in fewer product recalls, improved batch consistency, and greater confidence in the quality of the medication supply.
Patients should always be the guiding light in pharmaceutical manufacturing. They deserve safe and effective treatments. A return to domestic production would enhance quality assurance. Improving it every step of the way, from raw material sourcing to final packaging.
Stronger National Security
Pharmaceutical products are a cornerstone of national health and security. When production is concentrated overseas, vulnerabilities become more apparent. Whether it is interruptions to supply or trade restrictions, or foreign political instability, we have more challenges to the health and security. In times of crisis, foreign governments may prioritize domestic needs and restrict exports of critical medications.
Now let's look at that risk when considering essential medications such as antibiotics, vaccines, and insulin. The lack of domestic manufacturing capacity limits the nation's ability to respond to emergencies. If there is another pandemic, or there are bioterrorism threats, or a natural disaster, reshoring pharmaceutical CMOs will strengthen national security by reducing dependence on international suppliers. This will allow for faster production of essential drugs in response to urgent needs. We need to mitigate the vulnerability before any of these disasters strike. With a domestic manufacturing infrastructure in place, as a result, the United States, or even the Americas, will be able to better protect its citizens during emergencies and avoid the harmful effects of drug shortages.
Economic Growth and Job Creation
Potential for economic development is another major advantage of bringing CMOs back to the United States. The pharmaceutical industry is a sector that is growing and expanding. This vital industry can provide high-paying jobs in science, engineering, quality control, and logistics.
Local communities are economically stimulated in related industries, including transportation, utilities, and construction. Building new manufacturing facilities or expanding existing ones could create employment opportunities for both skilled and entry-level workers. As more companies invest in domestic production, entire ecosystems develop around pharmaceutical hubs. These ecosystems create long-term economic benefits that go beyond the companies themselves.
In regions facing economic decline, pharmaceutical manufacturing plants have the potential to provide a much-needed economic boost. The jobs that are created tend to have better wages and benefits than many other industries, contributing to a higher standard of living. This, in turn, creates community stability.
Increased Transparency and Accountability
Patients, providers, and regulators must know where medications are produced and under what conditions. Transparency is essential. When production is moved overseas, transparency often decreases.
Domestic manufacturing encourages greater openness. Regulatory agencies have greater ease of access to inspect facilities and review records. Companies can communicate more clearly with the public about sourcing, safety, and compliance. This builds trust between the pharmaceutical industry and the patients it serves.
Consumers are showing interest in where their medications are made. Just as people care about the origin of their food, many want to know whether their medicines are produced safely and ethically. Reshoring supports this desire for greater accountability and corporate responsibility.
Technological Advancements and Innovation
When pharmaceutical manufacturing is brought back to the United States, there is a greater opportunity for innovation. Continuous manufacturing, advanced automation, and improved quality control systems are all more likely with a chain of domestic facilities. They are more likely to adopt cutting-edge technologies. These technologies increase efficiency, reduce costs over time, and enhance product consistency.
In contrast, many overseas facilities are slower to modernize due to limited capital investment or regulatory restrictions. Reshoring CMOs allows American firms to lead in pharmaceutical technology and manufacturing science.
Collaboration is strengthened: manufacturers, research institutions, and universities work together more naturally. The exchange of knowledge and technology accelerates innovation and shortens the time needed to bring new treatments to market.
Resilience in Times of Crisis
We have seen how vulnerable the global pharmaceutical supply chain can be. Recent events have led to delays, shortages, and rising prices. When companies rely too heavily on foreign suppliers, they lose the ability to adapt quickly to changing circumstances.
Creating a network of CMOs domestically increases resilience. Manufacturers will be able to launch emergency initiatives in a timely manner. They can adjust production levels or shift resources without waiting for overseas partners. This flexibility is essential during times of national crisis.
By investing in domestic capacity now, pharmaceutical companies can ensure they are prepared for the challenges of tomorrow. Reshoring is a long-term strategy that increases preparedness and stability.
Ethical and Environmental Considerations
Ethical labor practices and environmental standards can vary widely across different countries. CMOs may or may not operate under conditions that do not align with US values. There might be extremely low wages, unsafe working conditions, or limited environmental protections.
Bringing pharmaceutical manufacturing back to the US ensures compliance with fair labor laws and environmental regulations. Companies are required to provide safer working conditions and reduce their environmental impact. And consumers, in this case, patients, are increasingly interested in how products are made. These efforts support sustainability goals and improve corporate reputation.
Ethical sourcing and responsible production practices are no longer optional. Reshoring aligns with public expectations and supports the broader goal of corporate social responsibility.
I hope that after reading this, we all can agree that the decision to bring pharmaceutical CMOs back to the United States is both strategic and responsible. Offshore manufacturing has seemed to offer short-term cost savings. At the same time, it has created long-term risks related to quality, supply chain stability, and national security.
By investing in domestic production, the pharmaceutical industry can strengthen its foundation. Advantages include more reliable supply chains, enhanced quality control, stronger national security, economic growth, technological leadership, and ethical transparency.
Reshoring is certainly not without its challenges; it requires capital investment, workforce development, and regulatory planning. The long-term benefits do outweigh the initial costs. We can deliver safer, more reliable treatments to the people who need them most by producing more of our medications closer to home. And our industry will do all of that with a smaller footprint.
As the pharmaceutical industry faces growing complexity and rising public expectations, reshoring CMOs is a powerful step toward a more secure, transparent, and innovative future. The time has come to rebuild U.S. pharmaceutical manufacturing, for both economic reasons and the health and well-being of the nation.
If you are in a position to contract your organization's CMO and would like to discuss how to reshore manufacturing, please contact us at
hello@metisconsultingservices.com
Or for more information, see our website at:
https://www.metisconsultingservices.com/
Or better yet, schedule an appointment:
https://calendly.com/sbradley-metisconsultingservices